Molecular determinants of differential host susceptibility to SARS-CoV-2 at the point of entry
CORONAmem
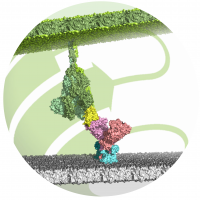
In the first step of the SARS-CoV-2 infection process, the ACE2 receptor/B0AT1 complex (pink/light blue) on the human cell membrane (grey) is recognized and bound by the viral spike protein (S-protein) (green/yellow). This leads to a fusion of viral and human membranes, allowing the virus to replicate its genome in the cell and the infection to progress. The receptor binding domain (RBD) of the S-protein is shown in yellow.
(Image: designed by Prof. Dr. Milton T. Stubbs)
One of the most striking features of the Covid-19 outbreak is the enormous variation in individual susceptibility to the virus. Coronaviruses gain access to the host cell by binding host membrane proteins via the viral membrane envelope spike protein (S-protein). They represent a Trojan horse that allows infection after interaction of the S-protein with the host cell.
One goal of this proposal is to characterize the interactions of the S-protein with genetic variants of host membrane proteins, both structurally and biophysically. The knowledge gained will allow us to better understand why individuals respond so differently to the virus. This may allow classification of at-risk patients and pave the way for personalized therapies.
Since the lipid composition of the host cell depends on the tissue as well as on the age, dietary habits and previous diseases of the host, studies on the dependence of the membrane binding of the spike protein on the lipid composition could provide insights into why certain populations are more susceptible to infection with SARS-CoV-2, regardless of their genetic background. Therefore, the interactions of the S-protein with model membranes will be investigated to analyze the influence of lipid composition on protein-membrane interactions.
Key Aspects
The work in the project has three interwoven key aspects: (A) the identification, cataloging, and mapping of the genomic variants of the ACE2-receptor and the SARS-CoV-2 S-protein; (B) the study of the structure of biomolecular complexes formed by the SARS-CoV-2 S-protein and the ACE2-receptors; and (C) an analysis of the interactions of the spike fusion peptide with model membranes to determine the influence of the lipid composition of the membranes.
Instruments
information coming soon
Selected Publications
Kyrilis FL, Belapure JS, Kastritis PL. (2021)
Detecting Protein Communities in Native Cell Extracts by Machine Learning: A Structural Biologist’s Perspective. Front. Mol. Biosci. 8:660542. doi:10.3389/fmolb.2021.660542
Group Members

project leader
Prof. Dr. Milton T. Stubbs
Executive director of HALOmem since 2008
Institute of Biochemistry and Biotechnology
Faculty of Natural Sciences I
Martin Luther University Halle-Wittenberg
http://www.biochemtech. uni-halle.de/xray/

collaborating professor
Prof. Dr. Kirsten Bacia
Institute of Chemistry
Faculty of Natural Sciences II
Martin Luther University Halle-Wittenberg
http://www.chemie.uni- halle.de/bereiche_der_chemie/physikalische_chemie/prof_bacia/

collaborating professor
Jun.-Prof. Dr. Panagiotis L. Kastritis
Specialist in Cryo-Electron Microscopy and Computational Structural Biology
IWE ZIK HALOmem / Institute of Biochemistry and Biotechnology
Faculty of Natural Sciences I
Martin Luther University Halle-Wittenberg
https://blogs.urz.uni-halle.de/kastritislab/

scientist
Dr. Andrea Scrima
phone
+49 345 5524945
e-mail
andrea.scrima(at)chemie.uni-halle.de

scientist
Jan Ebenhan
phone
+49 345 5524956
e-mail
jan.ebenhan(at)chemie.uni-halle.de

scientist
Dr. Jaydeep Sanjay Belapure
phone
+49 345 5524985
e-mail
jaydeep.belapure(at)bct.uni-halle.de

scientist
Dr. Alaa Shaikhqasem
phone
49 345 5525826
e-mail
alaa.shaikhqasem(at)biochemtech.uni-halle.de

master student
Sascha Mrachacz
phone
+49-345-55-24905 (Lab)
e-mail
sascha.mrachacz(at)student.uni-halle.de
associate PhD student
Marija Sorokina


